About Ethers and Oxides Powerpoint Presentation
Download Ethers and Oxides Powerpoint (US letter size)
Download Ethers and Oxides Powerpoint (A4 paper)
About Ethers and Oxides
Are these components ethers or oxides? In essential oil chemistry, there’s significant confusion between these terms due to historical naming conventions that don’t align with precise chemical definitions. Most components called “oxides” in essential oil chemistry are technically ethers, specifically cyclic ethers – compounds where an oxygen atom is incorporated into a ring structure (C-O-C bonds within a ring). The term “oxide” in essential oil nomenclature is largely a historical convention rather than precise chemical terminology.
ETHERS
Ethers are organic compounds in which an oxygen atom connects two carbon atoms through single bonds (C-O-C). They can be either acyclic (open chain structure, R-O-R’) or cyclic (oxygen incorporated into a ring structure), and both types are commonly found in essential oils as volatile, aromatic components.
Ethers are organic compounds where an oxygen atom connects two carbon atoms (C-O-C):
- Acyclic ethers: Open chain structure (R-O-R’)
- Cyclic ethers: Oxygen incorporated into a ring structure (4, 5, 6+ membered rings)
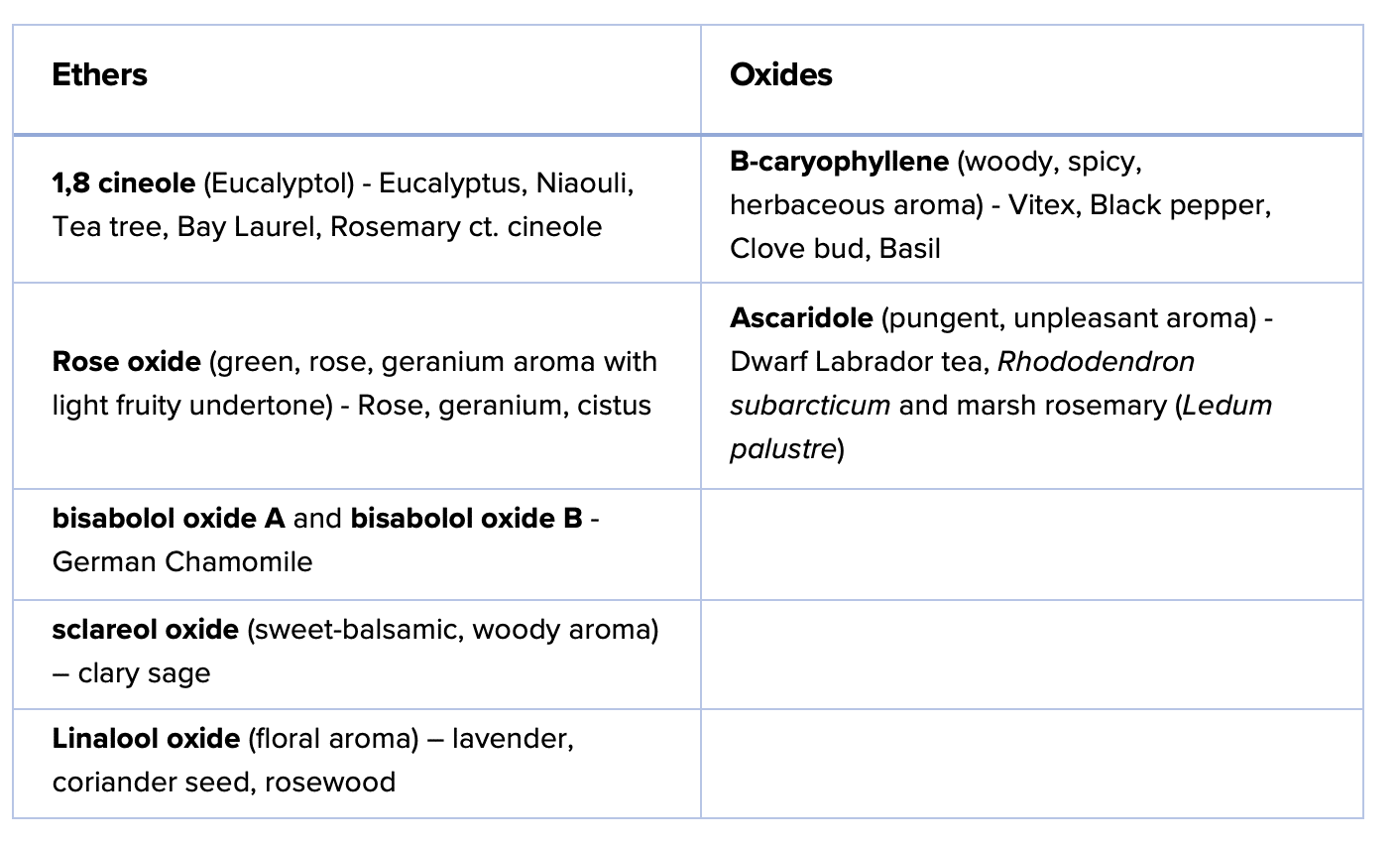
Examples of CYCLIC ETHERS incorrectly called “oxides”:
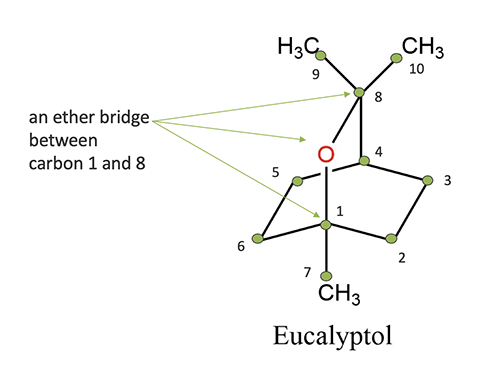
- 1,8-Cineole (eucalyptol) – bicyclic ether, 6-membered ring with oxygen
- Rose oxide – cyclic ether with 6-membered ring with oxygen
- Linalool oxide – cyclic ether
- Sclareol oxide – Sclareol oxide contains a pyran ring (6-membered cyclic ether)
OXIDES
In organic chemistry, true oxides include epoxides (3-membered rings containing one oxygen atom) and peroxides (compounds containing an oxygen-oxygen bond, R-O-O-R), both of which are significantly more reactive than larger cyclic ethers due to ring strain or bond instability.
True Organic Oxides include:
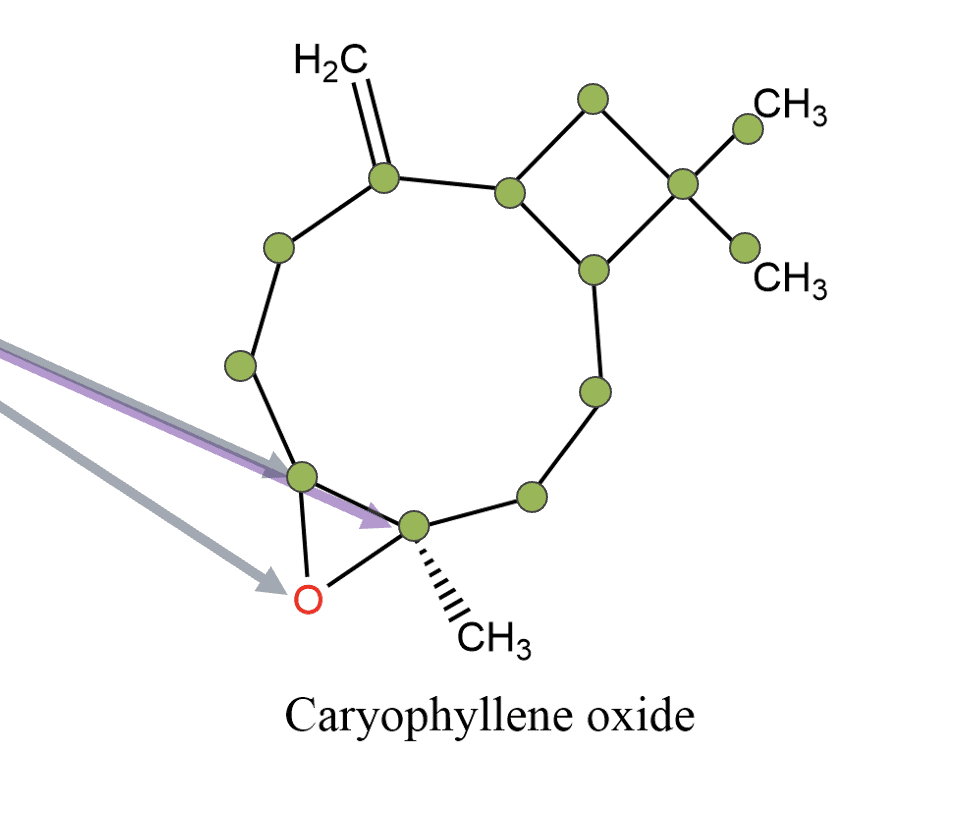
- Epoxides: 3-membered rings containing one oxygen (also called oxiranes), e.g. b-caryophyllene oxide
- Peroxides: Contain an oxygen-oxygen bond (R-O-O-R)
Examples of TRUE OXIDES:
- β-caryophyllene oxide – contains an epoxide ring (3-membered)
- Ascaridole – contains a peroxide bridge (O-O bond)
Key Distinction:
Epoxides (3-membered rings) are chemically oxides. Larger cyclic ethers are just ethers, despite often being called “oxides” in essential oil nomenclature. Both can appear in essential oils, have similar volatility, and contribute to aroma, but their chemical classification is different.
Food for thought:
The term “oxide” commonly used in aromatherapy literature to describe compounds like β-caryophyllene oxide, linalool oxide, and bisabolol oxides likely stems from the practical observation that these molecules are oxidation products of their parent terpenes, rather than from strict chemical nomenclature. When essential oil components containing carbon-carbon double bonds are exposed to atmospheric oxygen—either naturally in the plant or post-distillation during storage—oxygen atoms are added to these vulnerable sites, frequently forming cyclic ethers or epoxide or peroxide (true oxide) components.
Ethers/oxides compounds have names which end in -oxide or
in the case of eucalyptol -ol.
Ether & Oxide Physical Characteristics
- Polarity: Both are moderately polar (less than alcohols, more than hydrocarbons)
- Solubility: very slightly soluble in water, soluble in non-polar solvents such as vegetable oils
- Volatility: ethers are highly volatile; oxides are volatile depending on structure
- Boiling point: lower boiling point than alcohols of similar structure
- Aroma: variable aroma strength, giving characteristic aromas to the essential oils in which they occur, even at percentages as low as 0.3% (for example, rose oxide in Rosa damascena oil)
- Color: colorless
Examples of Ethers and True Oxides
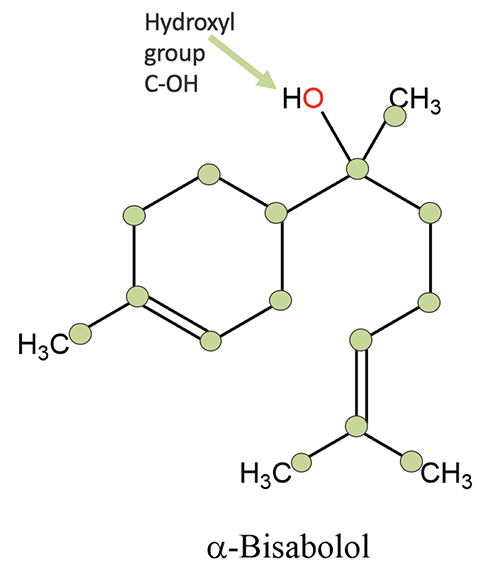
NOTE: The ethers are a unique chemical family in that it is hard to generalize about the whole family. The main ether component found in essential oils is eucalyptol. Most of the other oxides are found as minor components of essential oil, generally at less than 1 to 3%, with the exception being the bisabolols, which can occur as major components of German chamomile essential oil. We will focus our attention on eucalyptol in the sections that follow.
General therapeutic actions of eucalyptol:
-
- Analgesic
- Anti-inflammatory
- Antiviral (respiratory)
- Antibacterial (respiratory)
- Powerful Expectorant
- Decongestant (respiratory)
- Mucolytic
- Immunomodulatory
- Preventative to respiratory disorders
- Supportive to the respiratory system
Eucalyptol-rich essential oils
1,8 cineole rich essential oils:
- Eucalyptus radiata: 65-75%
- Eucalyptus globulus: 72.71-85.82%
- Eucalyptus polybractea: 60-95%
- Eucalyptus dives: 60-75%
- Eucalyptus smitthi: 70-80%
- Laurel (Laurus nobilis): 31.9-58.59%
- Myrtle ct cineole (Myrtus communis): 50+%
- Niaouli ct. cineole (Melaleuca quinquenervia): 49-62%
- Ravintsara ct. cineole (Cinnamomum camphora): 50-68%+
- Rosemary ct. cineole (Salvia rosmarinus): 19-37+%
Safety notes for eucalyptol-rich essential oils
- Ether components are relatively non-toxic when used appropriately.
- Keep eucalyptol-rich essential oils away from infants and children. Numerous case reports reveal the high incidence of children unintentionally taking eucalyptol-rich essential oils internally. Adverse reactions may include epigastric pain, vomiting and CNS symptoms, burning sensation in the mouth and throat, spontaneous vomiting, respiratory difficulty, and seizures.3,4
- Avoid applying eucalyptol-rich essential oils to the face or nose of infants or young children (under age 5). Do not instill in nose of infants or young children (e.g. via nasal spray).
- Do not use eucalyptol-rich essential oils orally with children under three.